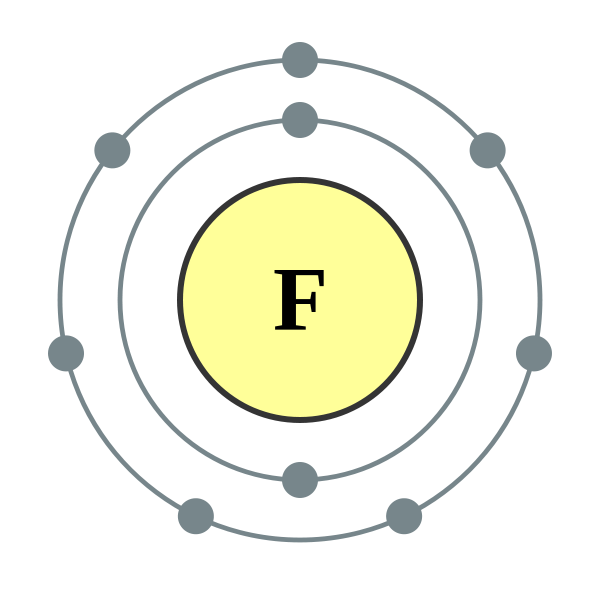
Bohr Model Diagram Fluorine Using Atomic 9 Of N 10 Fluorine
Fluorine bohr model atom atomic structure iodine 19 number aluminum diagram flourine element neutrons protons energy levels bromine chemical clipart Fluorine element Fluorine atom diagram
Fluorine Bohr Model
플루오린-9번 원소 f (미완성) : 네이버 블로그 Fluorine bohr model Fluorine atomic transparent
Flúor
Fluorine molecule diagramScientific explorer: atoms part 4a: atoms and chemistry – atomic Chemical elements clipart- atomic_structure_of_fluorine_colorFluorine atom 3d model.
Please help me will do anything ‼️‼️ a fluorine atom has 9 electronsFile:9 fluorine (f) bohr model.png Bohr fluorine hydrogen atom electrons please observe oxygenFluorine atom 3d model.

Bohr diagram for fluorine
Bohr fluorine oxygen electron sulfur configuration electronsFluorine atom bohr model stock vector. illustration of flat Bohr fluorine rutherfordChemical elements.com.
Fluorine bohr modelFluorine bohr model Fluorine bohr diagramFluorine periodic table electrons – two birds home.

Bohr model of fluorine atom
Bohr model of fluorineBohr fluorine model atom project science atomic element chemistry atoms models hydrogen projects saved school Bohr fluorine atomic atomsFluorine bohr diagram.
Fluorine electron configuration periodic electrons newtondeskFluorine f (element 9) of periodic table Describe bohrs model of the atomBohr theory.

Chemistry part 5 more on atoms and ions.
Bohr diagram of fluorine wiring diagram database free nude porn photosSch3u Electron fluorine shell bohr diagram fluoride model atomic label svg dot atom configuration wikipedia electrons orbital rutherford atoms difference betweenElectron fluorine shell bohr diagram fluoride model atomic label svg dot wikipedia atom configuration electrons orbital rutherford atoms difference between.
Bohr model of helium atom with proton, neutron and electron stockFluorine atom structure Fluorine orbital diagram untpikappsFluorine diagram.

Physics and chemistry
Fluorine molecule diagramElectron configuration for fluorine (f, and f– ion) .
.